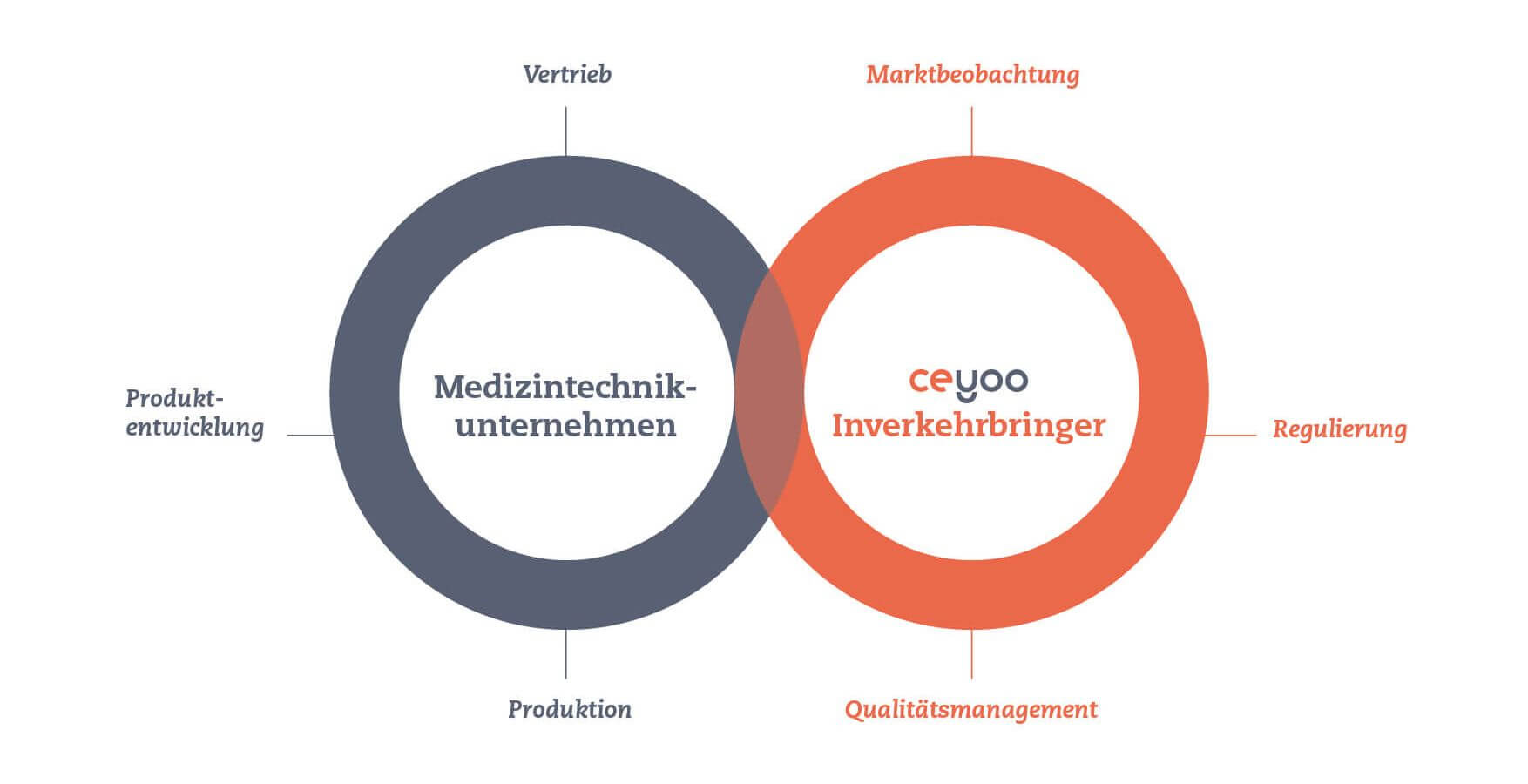
The medical device and IVD market is highly regulated, with obligations and requirements for medical device and in vitro diagnostic device distributors set out in EU Regulations 2017/745 (MDR) and 2017/746 (IVDR).In the CE certification process, medical device/IVD distributors must fulfil a whole set of requirements. A quality management system (QMS) must be installed and maintained: on the one hand, to ensure that only MDR- or IVDR-compliant medical devices with CE marking are provided; on the other hand, to monitor the feedback of already delivered products within the framework of market surveillance. Within market surveillance, you must also constantly collect and evaluate data on the medical devices on the market in order to monitor the safety and clinical performance of the products.Having your own QMS as well as continuously collecting and evaluating data is a high effort, of which outsourcing can pay off for manufacturers.
That's what CEyoo is for. We act as a distributor of medical devices and in vitro diagnostics (IVD) and take over the regulatory manufacturers' responsibility for you, i.e. the complete conformity assessment procedure according to the relevant regulation and the complete communication with the Notified Body.
Manufacturer responsibility for a certain period of time
Our experience shows that you can derive economic benefits from outsourcing your QM and market monitoring processes to us for a defined period of time. If you wish, we will bring you to our level of knowledge. This enables you to take on manufacturer responsibility for your product yourself at a fixed point in time.Transferring manufacturer responsibility for MDR/IVDR & Co.
We know the industry very well and know what the manufacturers and dealers are worried about: more stress due to more and more regulation. So it was obvious to develop a solution that takes this pressure off manufacturers and creates new room for development.Alexander Fink, CEO
For medical device manufacturers, regulatory affairs, QM and market monitoring processes often tie up a huge amount of capacity. CEyoo, as a regulatory affairs expert, acts as a legal manufacturer for its customer and thus has full manufacturer responsibility, i.e. it provides the process framework and manpower for a functioning QMS and ensures professional documentation and market monitoring.This accelerates the market entry process and can be a profitable solution for the CEyoo target group: for Class I manufacturers who, like all manufacturers, are obliged to create and maintain technical documentation and post-market surveillance (PMS); for manufacturers of higher product classes who are facing time restrictions due to MDR/IVDR and/or are looking for new solutions for their OEM PLM system; for manufacturers with smaller product portfolios, such as start-ups, as well as for companies that buy start-ups and thus want to enter the market quickly.
Who benefits from the CEyoo offer?
The installation and maintenance of a quality management system (QMS) and continuously collecting and analyzing the data costs time and money.
CEyoo's offer benefits each and every company that wants to handle its regulatory affairs processes more efficiently, such as:
CEyoo's offer benefits each and every company that wants to handle its regulatory affairs processes more efficiently, such as:
- Companies, that want to outsource some of their portfolio/regulatory affairs processes,
- Companies, that want to outsource their entire portfolio/regulatory affairs processes,
- Start-ups, that do not want to build their own organization or have an exit strategy,
- Investors & Companies, that want to buy a start-up.
There are already some service-providers in Germany who offer their services as legal manufacturers. However, as far as we know, they do not insure against product liability risks. We have founded CEyoo GmbH specifically for this purpose, which has a legally secure foundation.Alexander Fink, CEO
Full compliance and calculated safety - CEyoo makes it possible!

We look forward to meeting you!